
SOLVED: 116.5 g of oxalic acid dihydrate, H2C2O4 · 2H2O (MM 126.07 g/mol), is used to prepare 350.0 mL of solution 1? What is the molarity of a new solution made by

Oxalic acid, H2C2O4*2H2O molar mass = 12607 g/mol is often used as a primary standard for the sta - YouTube

What mass of oxalic acid dihydate, H2C2O4•2H2O, is needed to make a 0.498 M solution of oxalic acid in a 250.0 mL volumetric flask? - Quora



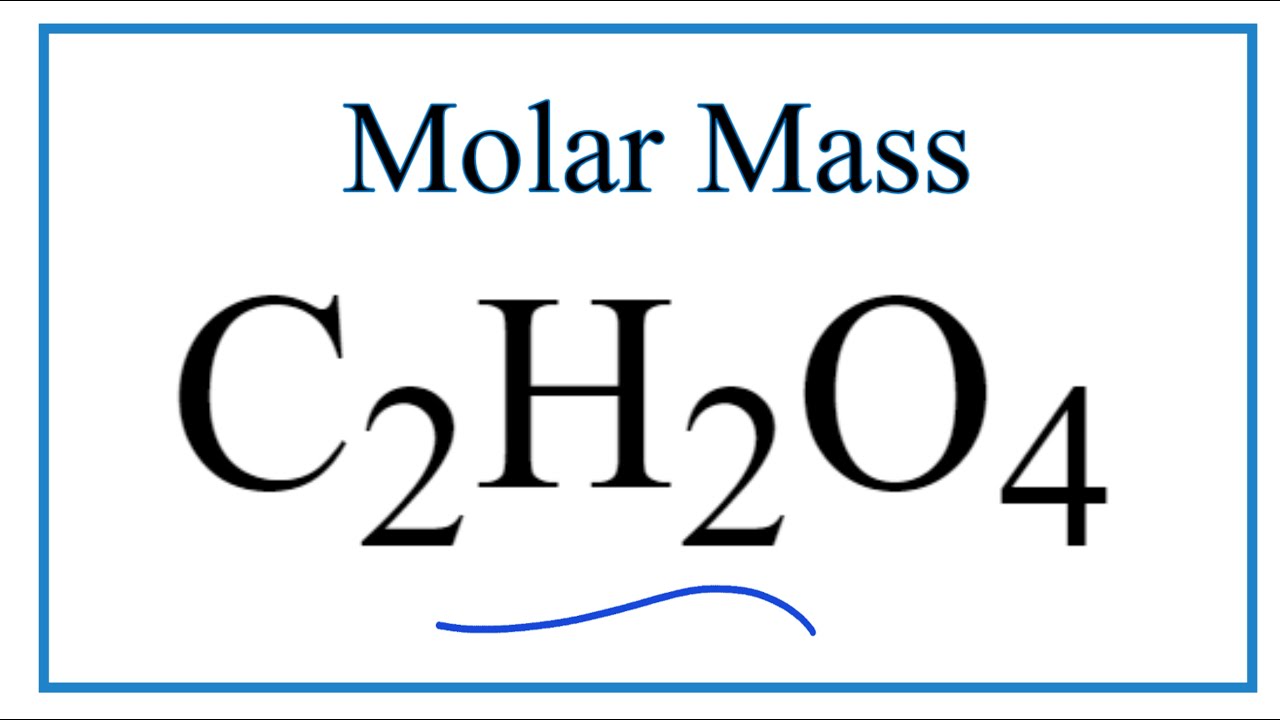



![Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun](https://cdn.shopify.com/s/files/1/0724/7981/products/15-005-1_1024x1024.jpg?v=1550179687)



