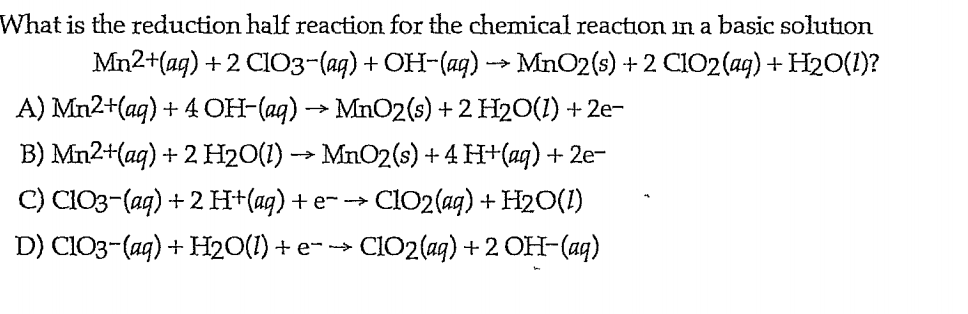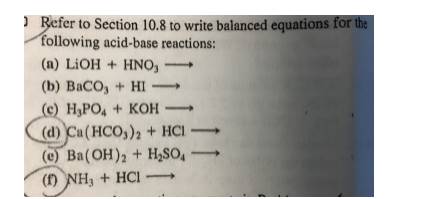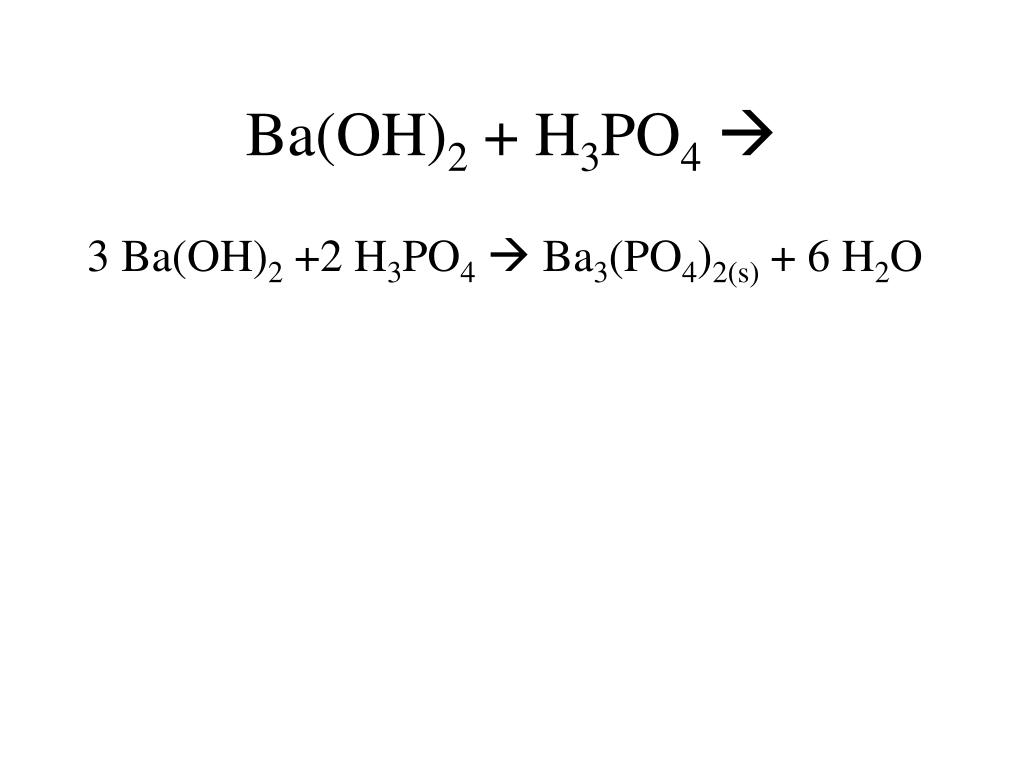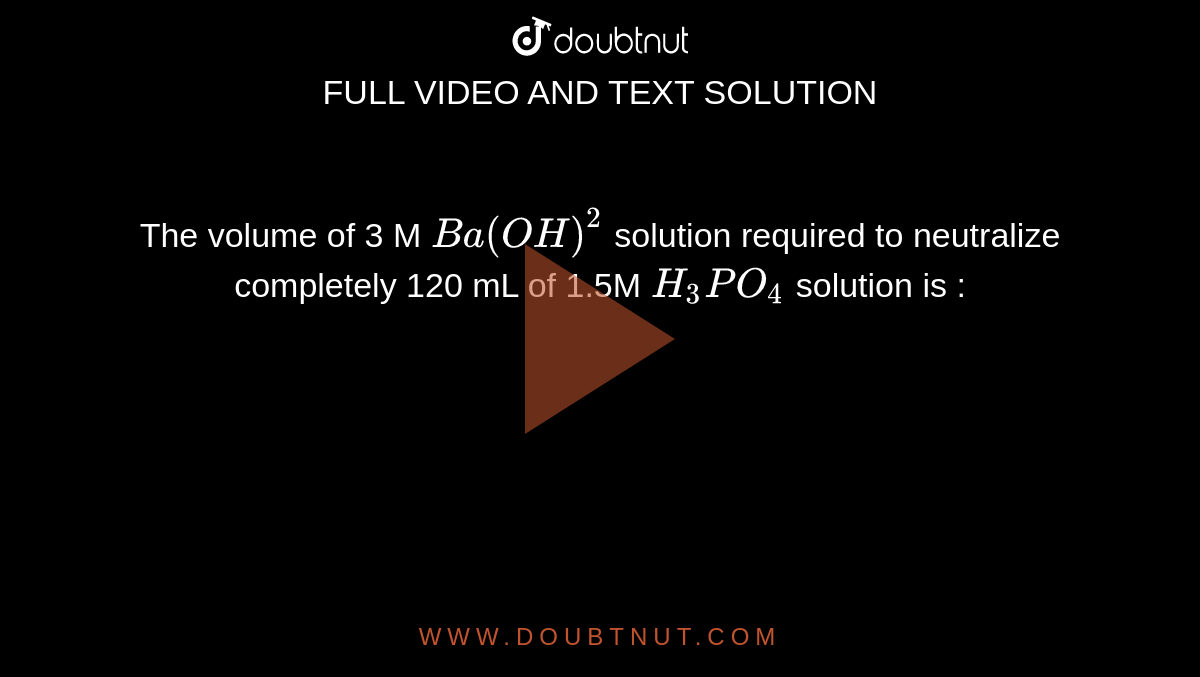
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :

Calculate the volume of 0.025 M Ca(OH)_2 solution which can neutralise 100 mL of 0.0001 M H_3PO_4. | Homework.Study.com
_how-to-write-the-net-ionic-equation-for-baoh2-h3po4-ba3po42-h2o.jpg)
How to Write the Net Ionic Equation for Ba(NO3)2 + Na3PO4 = Ba3(PO4)2 + NaNO3 from ba3 po3 2 Watch Video - HiFiMov.co
✓ Solved: What volume of 0.0521 M Ba(OH) 2 is required to neutralize exactly 14.20 mL of 0.141 M H 3...

OneClass: 8) the equations below, write the products for each reaction, and balance the For equations...

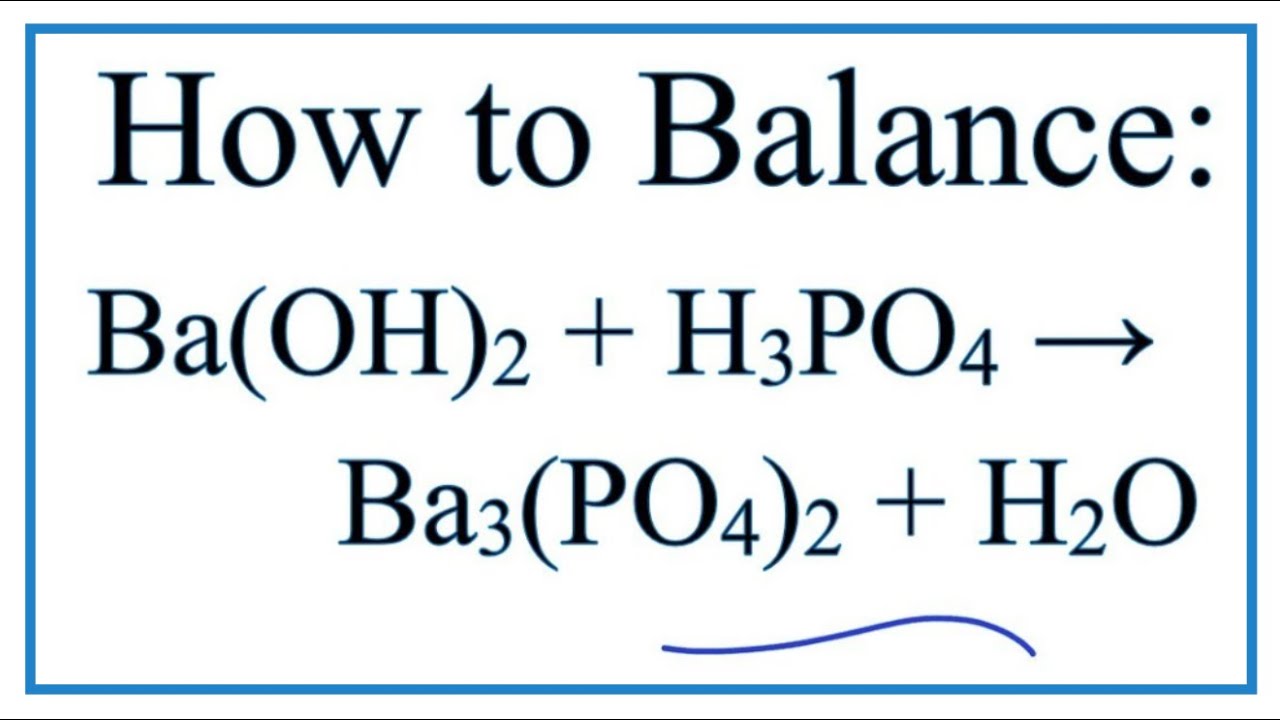
SOLVED: Barium hydroxide and phosphoric acid react as follows: 3 Ba(OH)2(s) + 2 H3PO4(aq) –> Ba3(PO4)2(aq) + 6 H2O(l) If 39.5 g of Ba(OH)2 are allowed to react with 51.0 g of