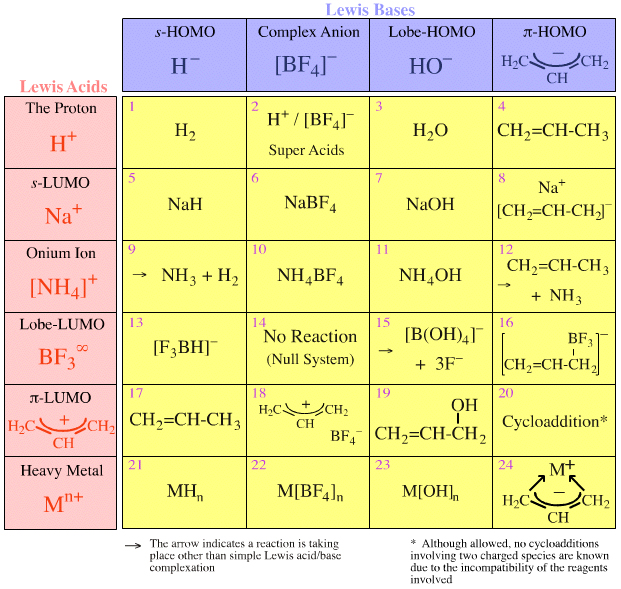
![SOLVED: You may find the Henderson-Hasselbalch equation useful when using buffers [conjugate base] pH pKa log1o [acid] (a) A buffer solution (Buffer 1) contains 0.110 molL-1 sodium hydrogen carbonate (NaHCO3) and 0.052 SOLVED: You may find the Henderson-Hasselbalch equation useful when using buffers [conjugate base] pH pKa log1o [acid] (a) A buffer solution (Buffer 1) contains 0.110 molL-1 sodium hydrogen carbonate (NaHCO3) and 0.052](https://cdn.numerade.com/ask_images/d656061ffc3f4a08a6b158ae3939f3b8.jpg)
SOLVED: You may find the Henderson-Hasselbalch equation useful when using buffers [conjugate base] pH pKa log1o [acid] (a) A buffer solution (Buffer 1) contains 0.110 molL-1 sodium hydrogen carbonate (NaHCO3) and 0.052

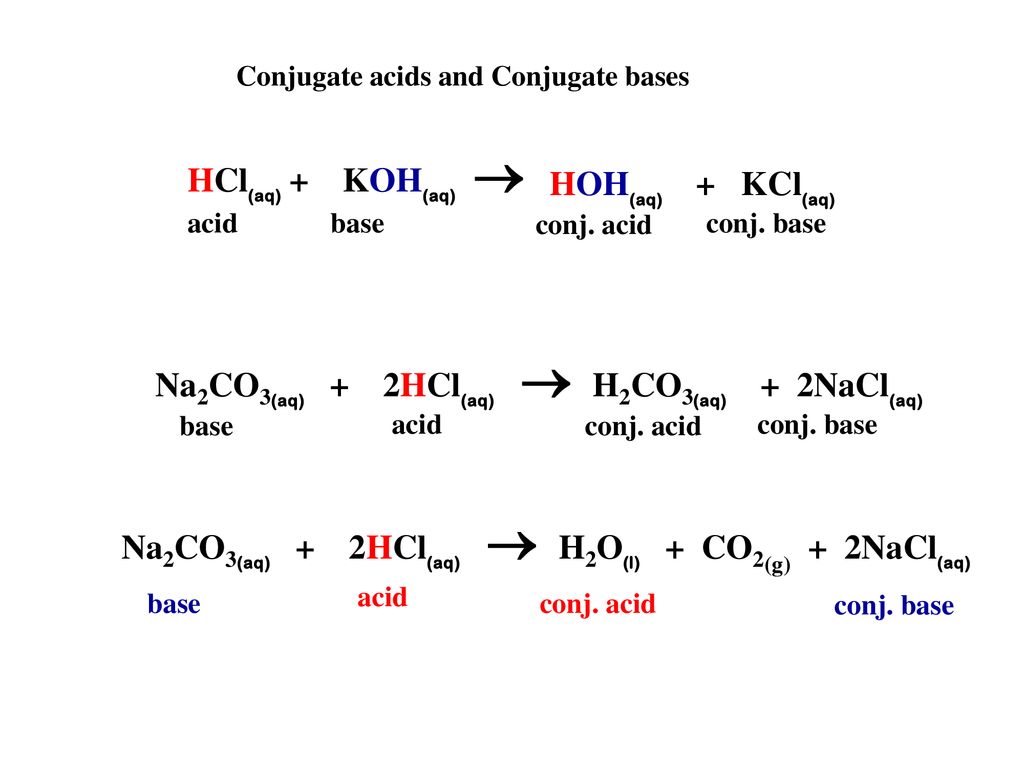
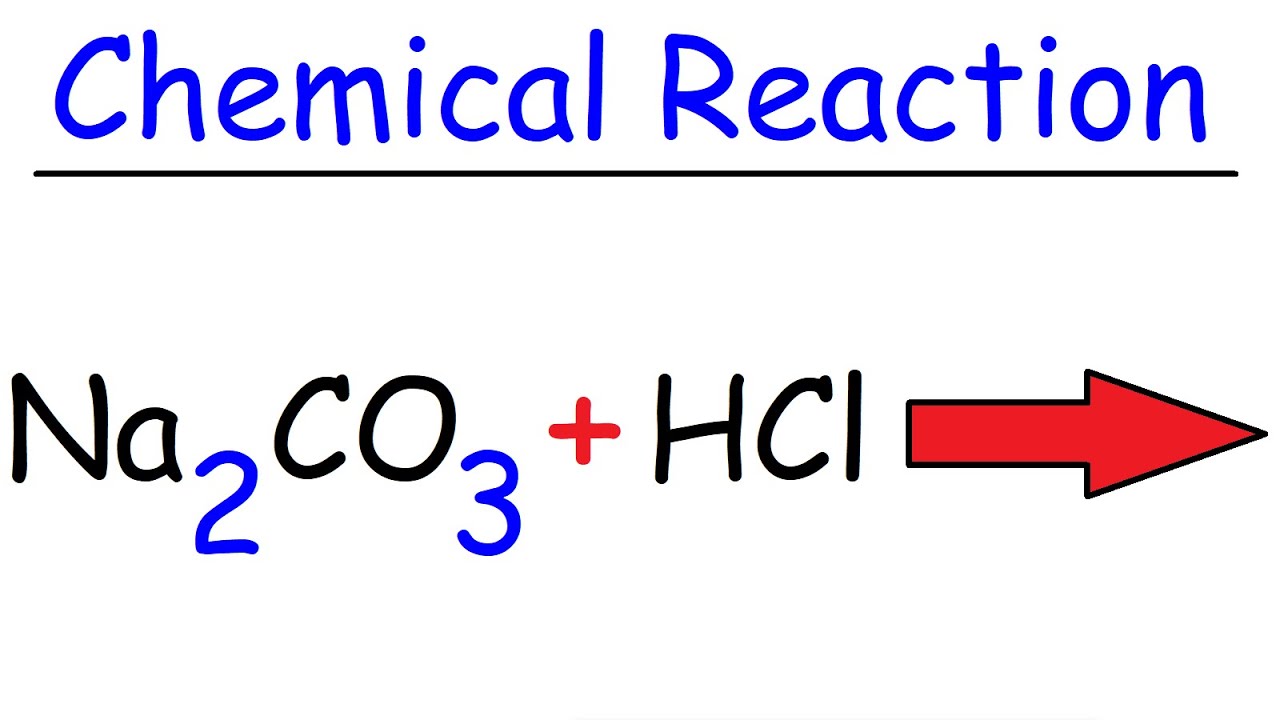
DOC) Standardization of a strong acid (HCl) with a weak base (Na2CO3).docx | Meharaj Ul Mahmmud - Academia.edu

Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram