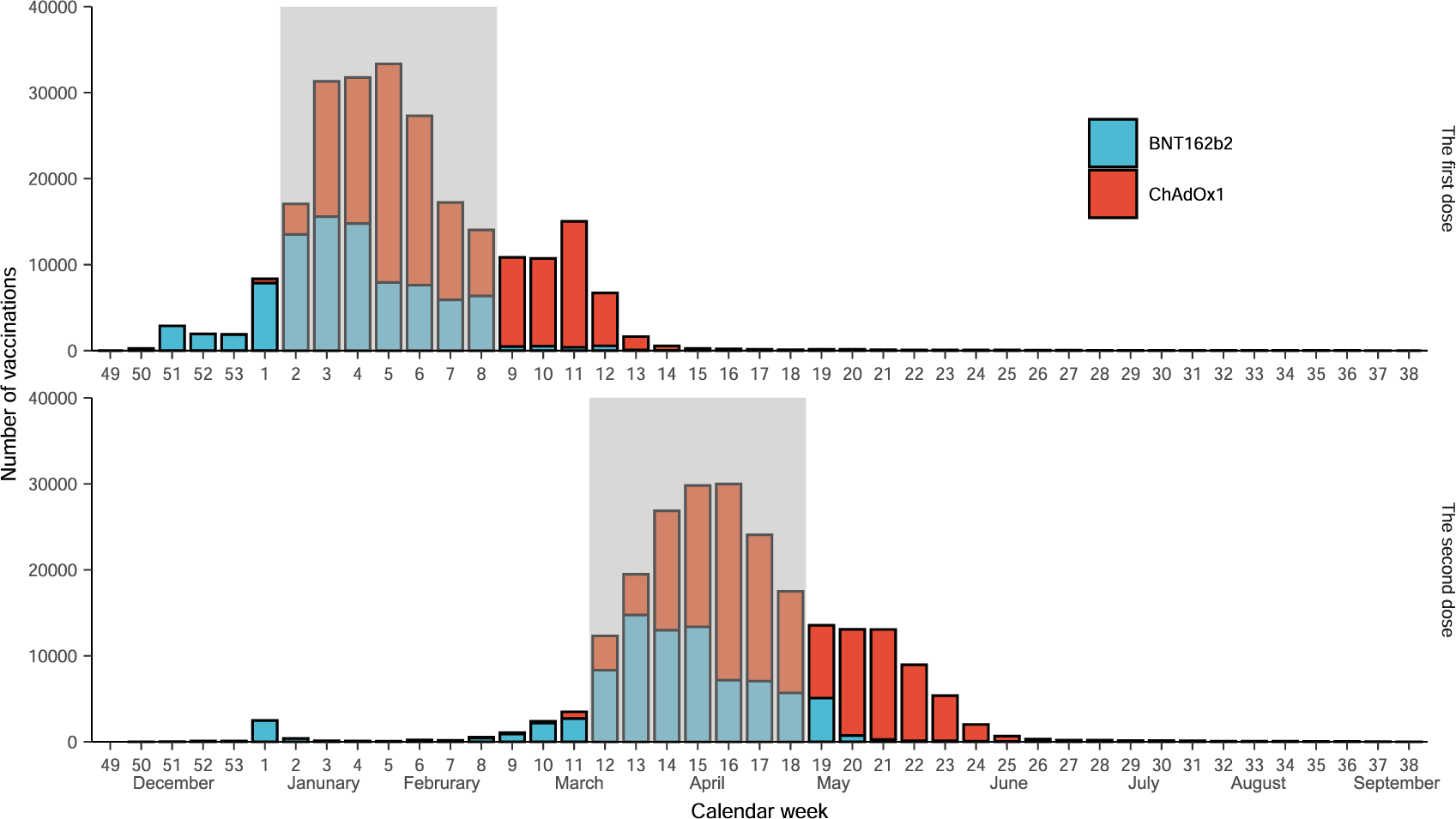
Comparative effectiveness of the BNT162b2 and ChAdOx1 vaccines against Covid-19 in people over 50 | Nature Communications
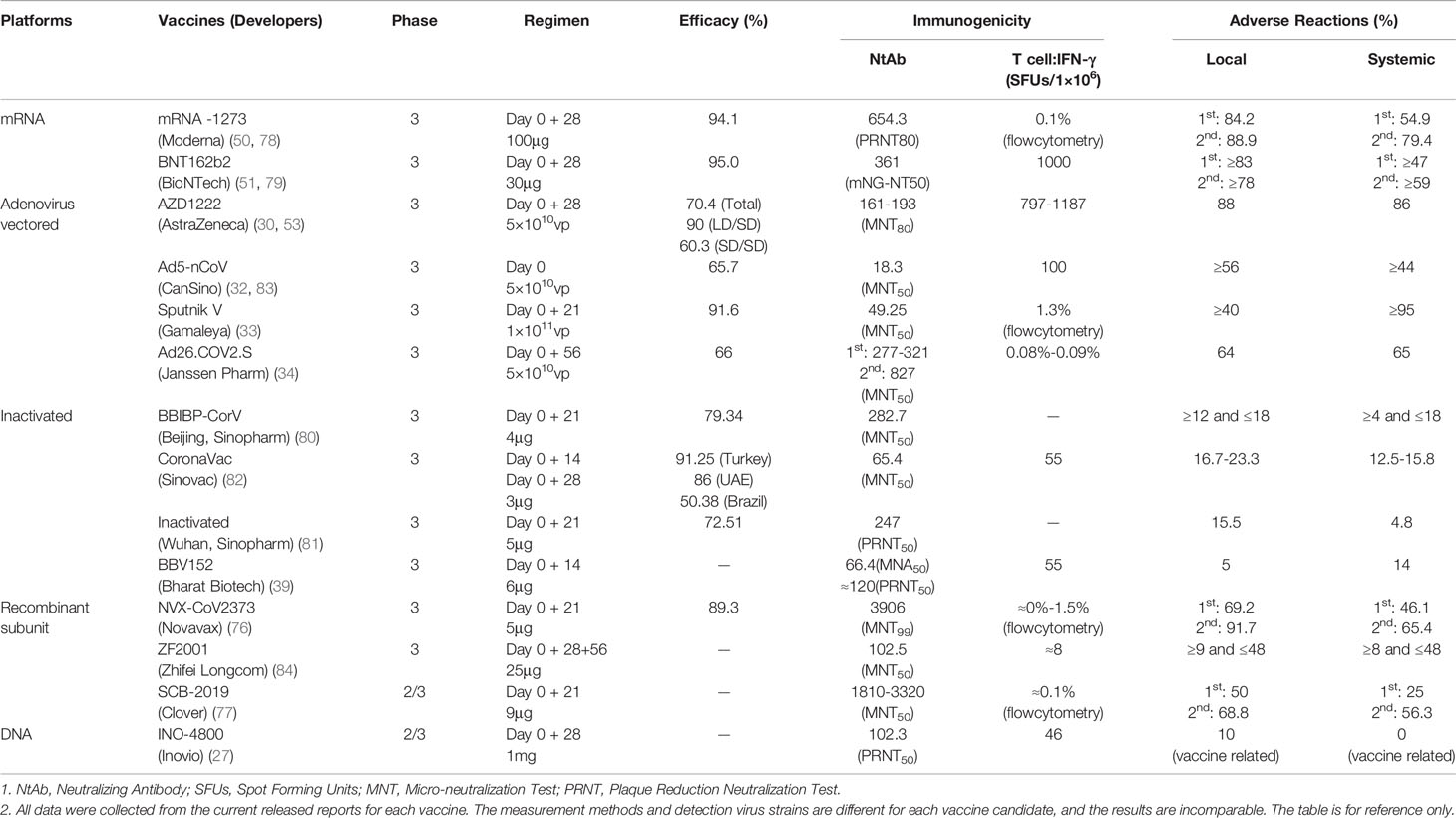
Frontiers | COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations

Article by cardiologist Aseem Malhotra made unsupported claims about the benefits and risks of COVID-19 vaccination - Health Feedback
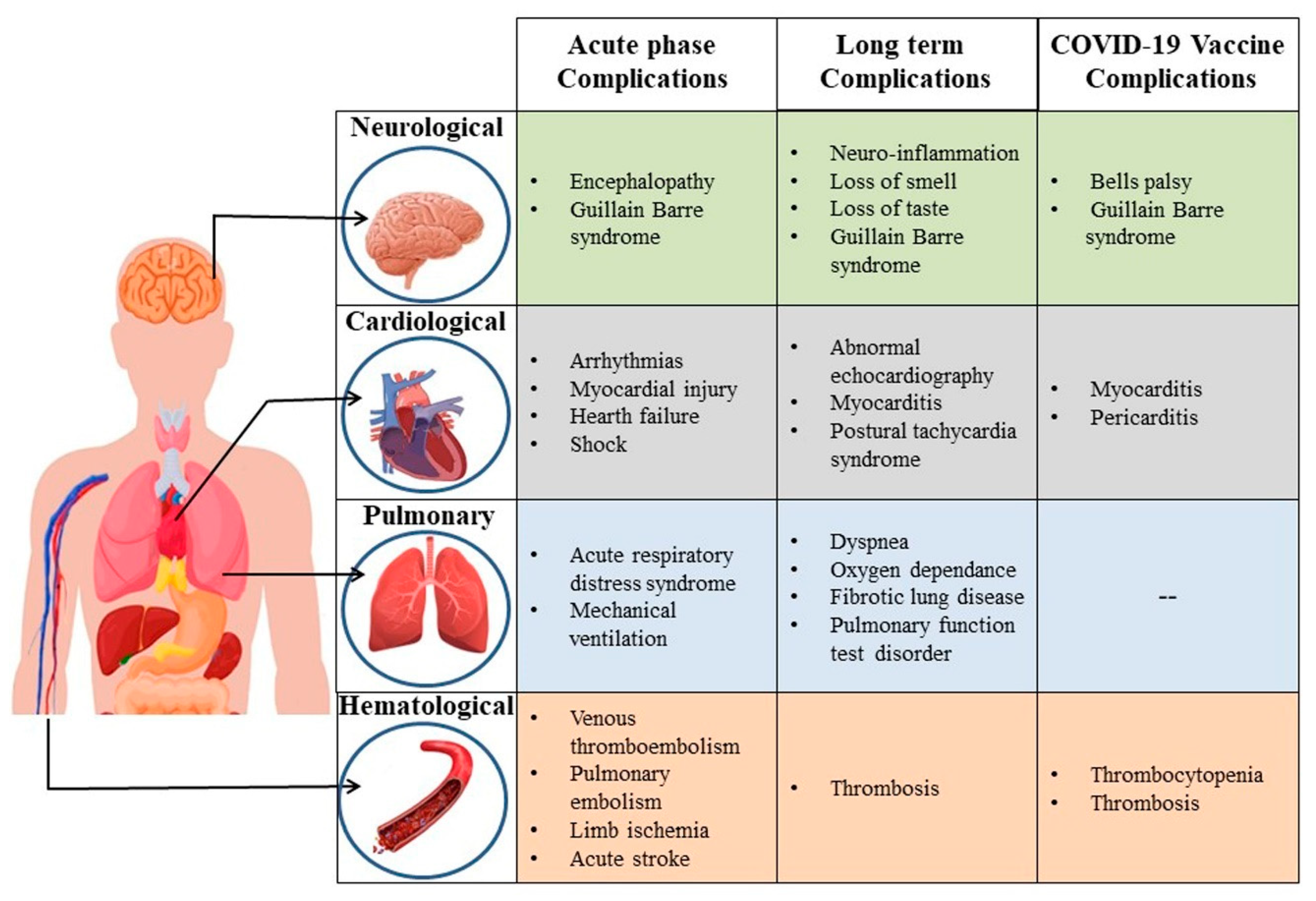
Vaccines | Free Full-Text | An Update on Complications Associated with SARS-CoV-2 Infection and COVID-19 Vaccination

Myths and Facts About the Vax — Debunking Common COVID-19 Vaccine Myths > TRICARE Newsroom > Articles

Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19–Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance — Nine States, June–August ...

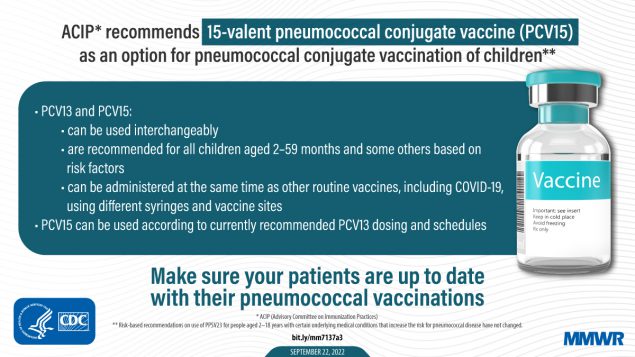
Use of 15-Valent Pneumococcal Conjugate Vaccine Among U.S. Children: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022 | MMWR
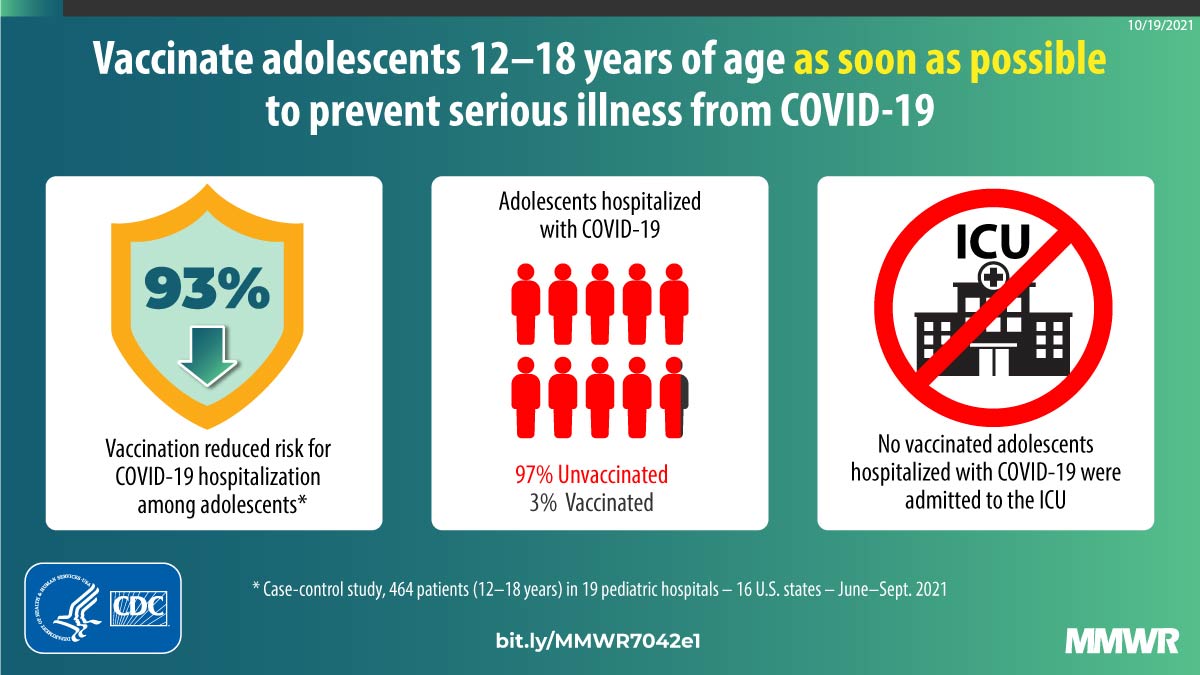
Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years — United States, June–September 2021 | MMWR